
MRI Safety Information
For Advanced Bionics Cochlear Implants, United States
Testing has demonstrated that the Advanced Bionics HiRes implant family is MRI Conditional. Conditions vary by geography. A patient with the implant may be safely scanned with MRI only under very specific conditions. Scanning under different conditions may result in severe patient injury.
MRI Safety Information by Type of Implant
| Type of Implant | Model Number | MRI Field Strength (T) | Spatial Gradient Field (T/m) | Max Head SAR (W/kg) | Max Body SAR (W/kg) | |
|---|---|---|---|---|---|---|
| Clarion 1.0* | MMT-5100 L/R | MRI is contraindicated | None | None | None | |
| Clarion 1.2* | AB-5100 L/R | MRI is contraindicated | None | None | None | |
| Clarion CII Bionic Ear* | AB-5100H | MRI is contraindicated | None | None | None | |
| HiRes 90K*, ** | CI-1400 | 1.5T | 2.5 T/m | ≤ 1 W/kg | ≤ 1.7 W/kg | |
| HiRes 90K Advantage*, ** | CI-1500 | 1.5T | 2.5 T/m | ≤ 1 W/kg | ≤ 1.7 W/kg | |
| HiRes Ultra | CI-1600 | 1.5T |
3.47 T/m | 13.90 T/m*** | ≤ 3.2 W/kg | ≤ 2 W/kg |
| 3.0T** | 6.9 T/m | ≤ 2.6 W/kg | ≤ 2 W/kg | |||
| HiRes Ultra 3D |
CI-1601 | 1.5T | 20 T/m | ≤ 3.2 W/kg | ≤ 2 W/kg | |
| 3.0T | 20 T/m | ≤ 2.6 W/kg | ≤ 2 W/kg | |||
* These devices are no longer sold in the EU or North America
** For MRI the magnet has to be removed surgically
*** With magnet removed
Select the Implant name below
for further MRI information
HiRes™ Ultra 3D Cochlear Implant



The implant components of the HiRes™ Ultra 3D cochlear implant are MR conditional.
The external components of the HiResolution™ Bionics Ear System are MR Unsafe and must be removed before entering a room containing an MRI scanner.
The HiRes Ultra 3D cochlear implants have a specifically designed magnet assembly that allows safe MRI scanning, without angular restrictions of the head, and without surgical removal of the magnet assembly or a bandaging protocol.
Do not allow patients with a HiRes cochlear implant to be in the area of an MRI scanner unless the following conditions have been met:
- The external sound processor and headpiece must be removed before entering a room containing an MRI scanner.
- Verify that the implant, or both implants if bilaterally implanted, are compatible for conducting an MRI before proceeding. Failure to do so can lead to device movement, device damage, multi-magnet assembly movement, patient discomfort, or trauma and pain to the patient.
- For other MRI situations, i.e. if the recipient has two different types of Advanced Bionics Cochlear Implants (e.g. HiRes Ultra 3D and HiRes 90k Advantage), the recipient must inform the MRI professional of the type of implants. If the recipient is not sure of the implant type, please contact the recipient’s cochlear implant clinic, the local AB office, or contact Advanced Bionics Technical Support at technicalservices@advancedbionics.com.
NOTE: MRI procedures are contraindicated for CLARION (C1 and CII) cochlear implant recipients. For information regarding MRI use with HiRes 90K, HiRes 90K Advantage, and HiRes Ultra cochlear implants, please contact Advanced Bionics Technical Support.
NOTE: MRI safety was evaluated only for the HiRes Ultra 3D. Interactions between non-Advanced Bionics implants and the HiRes Ultra 3D during MRI are unknown. - The recommended minimum duration of time post implant surgery prior to undergoing an MRI scan is 2 to 4 weeks in order to allow any inflammation to subside.
An MRI scan is not recommended if the patient has a fever.
CAUTION
- During the MRI procedure, you may experience pain, pressure, or discomfort. If this occurs, please notify your physician.
- Please consult with your physician prior to an MRI to determine if the benefits of an MRI are worthwhile over other imaging techniques.
- In case of two different implant types or models, MRI safety criteria that is most restrictive of the two implant types must be applied at the discretion of a qualified MRI professional.
Testing has demonstrated that the HiRes Ultra 3D cochlear implant is MR Conditional. Unilateral and bilateral recipients with this device with the multi-magnet assembly in place can be safely scanned in a horizontal closed bore quadrature coil MR system meeting the following conditions:
MRI Field Strength |
1.5T |
3.0T |
|---|---|---|
| Maximum Spatial Field Gradient | 20 T/m | 20 T/m |
| RMS Gradient Field | 34.4 T/s | 34.4 T/s |
| Peak Slew Rate | 200 T/m/s | 200 T/m/s |
| Maximum Whole Body Averaged SAR | 2.0 W/kg | 2.0 W/kg |
| Maximum Head Averaged SAR | 3.2 W/kg | 2.6 W/kg |
When tested under scan conditions defined above, the HiRes Ultra 3D cochlear implant produced a maximum temperature rise of <3˚C after 15 minutes of continuous scanning.
Note: During the scan, patients might perceive auditory sensations. Adequate counseling of the patient is advised prior to performing the MRI. The likelihood and intensity of the auditory sensations can be reduced by selecting sequences with a lower Specific Absorption Rate (SAR) and slower gradient slew rates.
In MRI testing, the measured range of device image artifact radius extending from the HiRes Ultra 3D cochlear implant for spin and gradient echo sequences and all imaging planes are as follows (refer to Tables A-B):
Table A: Artifact at 3.0T MRI Field Strength
Implanted |
Multi-magnet |
Artifact Range |
|---|---|---|
| Unilaterally | In Place | 5.5 - 6.9 cm |
| Unilaterally | Removed | 1.4 - 4.2 cm |
| Bilaterally | In Place | 6.1 - 7.4 cm |
| Bilaterally | Removed | 1.9 - 6.9 cm |
Table B: Artifact at 1.5T MRI Field Strength
Implanted |
Multi-magnet |
Artifact Range |
|---|---|---|
| Unilaterally | In Place | 4.1 - 6.5 cm |
| Unilaterally | Removed | 2.4 - 3.2 cm |
| Bilaterally | In Place | 5.7 - 8.2 cm |
| Bilaterally | Removed | 3.4 - 4.1 cm |
Note: For cases that would clinically benefit from reduced device artifact (for example, some head or neck scans), the internal multi-magnet assembly is surgically removed and possibly replaced with a Temporary Non-Magnetic Plug before the recipient undergoes an MRI procedure. The HiRes Ultra 3D cochlear implant can withstand 5 replacement cycles.
For additional information regarding the use of an MRI scanner with a HiRes Ultra 3D Cochlear Implant, please contact Advanced Bionics Technical Support at technicalservices@advancedbionics.com.
HiRes™ Ultra Cochlear Implant
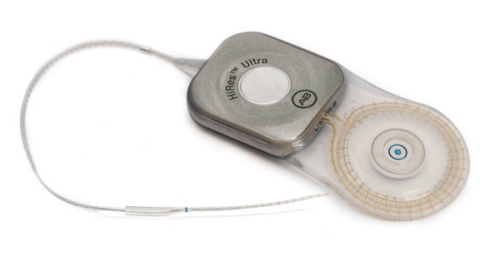
| Device | HiRes Ultra |
|---|---|
| Instructions For Use | HiResolution Bionic Ear System: HiRes Ultra |
| 1.5T magnet removed | Scanning ok under certain conditions |
| 1.5T magnet in place with MRI Antenna Coil Cover and bandaging protocol CI-7521 | Scanning ok under certain conditions |
| 3.0T magnet removed | Scanning ok under certain conditions |
- The internal magnet must be removed. See “Surgeon Manual for the HiRes Ultra Cochlear Implant” for removal instructions.
- The external sound processor and headpiece are MR Unsafe and must be removed before entering a room containing an MR scanner.
- Horizontal closed bore scanners with a static magnetic field of 3.0T
- Maximum spatial field gradient of 2,000 Gauss/cm (20 T/m)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of ≤ 2 W/kg at 3.0T for quadrature transmit RF body coils
- Maximum MR system reported, head averaged SAR of ≤ 2.6 W/kg at 3.0T for quadrature transmit RF head coils
- RMS gradient field of 30 T/s and peak gradient field of 150 T/s
Under the scan conditions defined above the HiRes Ultra implant is expected to produce a maximum temperature rise of <3°C after 15 minutes of continuous 3.0T scanning.
In MRI testing of unilateral and bilateral recipient conditions, respectively, the image artifact caused by the device extends from the HiRes Ultra implant approximately 5 cm using a spin echo pulse sequence in a 3.0T scanner MRI with the temporary non-magnetic plug in place. These artifacts may result in a loss of diagnostic information in the implant vicinity.
The recommended minimum duration of time post implant surgery prior to undergoing an MRI scan is 2 to 4 weeks in order to allow any inflammation to subside.
An MRI scan is not recommended if the patient has a fever.
- The internal magnet must be removed. See “Surgeon Manual for the HiRes Ultra Cochlear Implant” for removal instructions.
- The external sound processor and headpiece are MR Unsafe and must be removed before entering a room containing an MR scanner.
- Horizontal closed bore scanners with a static magnetic field of 1.5T.
- Maximum spatial field gradient of 2,000 Gauss/cm (20 T/m).
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of ≤ 2 W/kg at 1.5T (Normal Operating Mode) for quadrature transmit RF body coils.
- Maximum MR system reported, head averaged SAR of ≤ 3.2 W/kg at 1.5T (Normal Operating Mode) for quadrature transmit RF head coils.
- RMS gradient field of 30 T/s and peak gradient field of 150 T/s.
Under the scan conditions defined above the HiRes Ultra implant is expected to produce a maximum temperature rise of <3°C after 15 minutes of continuous 1.5T scanning.
In MRI testing of unilateral and bilateral recipient conditions, respectively, the image artifact caused by the device extends from the HiRes Ultra implant approximately 3 cm and 4 cm using a gradient echo pulse sequence in a 1.5T scanner with the temporary non-magnetic plug in place. These artifacts may result in a loss of diagnostic information in the implant vicinity.
The recommended minimum duration of time post implant surgery prior to undergoing an MRI scan is 2 to 4 weeks in order to allow any inflammation to subside.
An MRI scan is not recommended if the patient has a fever.
- The external sound processor and headpiece are MR Unsafe and must be removed before entering a room containing an MR scanner.
- A bandage must be applied using the bandaging protocol below.
- Horizontal closed bore scanners with a static magnetic field of 1.5T.
- Maximum spatial field gradient of 2,000 Gauss/cm (20 T/m).
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of ≤ 2 W/kg at 1.5T (Normal Operating Mode) for quadrature transmit RF body coils.
- Maximum MR system reported, head averaged SAR of ≤ 3.2 W/kg at 1.5T (Normal Operating Mode) for quadrature transmit RF head coils.
- RMS gradient field of 30 T/s and peak gradient field of 150 T/s.
Under the scan conditions defined above the HiRes Ultra implant is expected to produce a maximum temperature rise of <3°C after 15 minutes of continuous 1.5T scanning.
In MRI testing of unilateral and bilateral recipient conditions, respectively, the image artifact caused by the device extends from the HiRes Ultra implant approximately 8 cm using a gradient echo pulse sequence and >9.5 cm using a spin echo or gradient echo pulse sequence in a 1.5T scanner with the magnet in place. These artifacts may result in a loss of diagnostic information in the implant vicinity.
The recommended minimum duration of time post implant surgery prior to undergoing an MRI scan is 2 to 4 weeks in order to allow any inflammation to subside.
An MRI scan is not recommended if the patient has a fever.
Please note that the MRI Antenna Coil Cover CI-7521 and bandaging supplies must be on hand at the time of the MRI procedure. Please contact Advanced Bionics at customerservice@advancedbionics.com or 1-877-829-0026 prior to the MRI procedure to request these supplies.
Caution: The bandaging protocol with use of the MRI Antenna Coil Cover was developed and approved to prevent magnet displacement and counteract magnet torque during a 1.5T MRI procedure, but some discomfort and pain at the implant site may still be experienced. Please consult with your physician if this is an issue.
Caution: If discomfort persists following an MRI, please notify your physician.
Caution: Failure to secure the MRI Antenna Coil Cover and internal magnet in place during MRI may result in magnet displacement or the need for surgical revision.
Caution: Please consult with your physician prior to MRI to determine if the benefits of MRI are worthwhile over other imaging techniques.
Do not allow patients with a HiRes™ cochlear implant to be in the area of an MRI scanner unless the following conditions have been met:
- The bandaging protocol recommended by Advanced Bionics is followed when the patient undergoes an MRI procedure with the magnet left in place or,
- The internal magnet is surgically removed and possibly replaced with the Magnet Insert Dummy before the patient undergoes an MRI procedure.
- The external sound processor and headpiece are removed before entering a room where an MRI scanner is located.
- MRI preparations must be conducted outside of the MRI room.
- Verify that the implant, or both implants if bilaterally implanted, are compatible for conducting an MRI before proceeding. Failure to do so can lead to device movement, device damage, magnet movement, patient discomfort, or trauma and pain to the patient.
- MRI procedures are contraindicated for CLARION (C1and CII) Cochlear Implant recipients.
- Caution: The bandaging protocol with use of the MRI Antenna Coil Cover was developed and approved to prevent magnet displacement and counteract magnet torque during a 1.5T MRI procedure, but some discomfort and pain at the implant site may still be experienced. Please consult with your physician if this is an issue.
- Caution: If discomfort persists following an MRI, please notify your physician.
- Caution: Failure to secure the MRI Antenna Coil Coverand internal magnet in place during MRI may result in magnet displacement or the need for surgical revision.
- Caution: Please consult with your physician prior to MRI to determine if the benefits of MRI are worthwhile over other imaging techniques.
Before entering the area of an MRI scanner:
- Place patient in sitting position to allow access to the implant site.
Secure MRI Antenna Coil Cover over implant magnet site:
- Place the patient headpiece (with cable removed) over the implant site. The magnets will hold the headpiece in place.
- Cut a piece of the Coach bandage that is long enough to wrap around the head once.
- Wrap this piece around the head, so that the bandage covers the patient headpiece. Make this wrap tight.
- Outline the position of the headpiece on the bandage using a marker or pen.
- Slip out the patient headpiece, but keep the bandage in place.
- Slide the MRI Antenna Coil Cover under the bandage, lining it up with the marked outline of the headpiece.
Measure head size and bandage length needed for compression wrapping.
- Take the remaining Coach bandage roll and wrap the bandage around the head once, without stretching.
- Mark the location on the bandage that is one full wrap around the head. This is the head circumference.
- Unwrap the piece of bandage with the marked head circumference, and place it on a flat surface.
- Unroll the remaining bandage roll.
- Fold over the bandage start and crease at the marked head circumference.
- Cut or tear the remaining bandage where it overlaps with the bandage start. The resulting bandage piece length is twice the head circumference.
Apply compression bandaging:
- Wrap this cut piece, this time very tightly, by stretching the marked line an additional half turn around the head. This ensures 150% extension of the bandage.
- Continue wrapping the bandage at 150% extension for an additional 1.5 turns, resulting in 3 full turns total.
For additional information regarding the use of an MRI scanner with a HiRes Ultra device, please contact Advanced Bionics Technical Support at technicalservices@advancedbionics.com or 1.877.454.5051
Auditory Technical Services
Advanced Bionics provides one-on-one technical support for professionals – including surgeons, audiologists, clinicians, educators, and therapists.
